CHARLESTON, W.Va. — The West Virginia Department of Health and Human Resources (DHHR) has issued the following information on the state’s vaccination program.
COVID-19 VACCINATION BASICS
What role does COVID-19 vaccination play in helping to curb the pandemic?
COVID-19 vaccination will help protect ourselves and others from the disease and save lives. Vaccines both prevent and reduce severity of disease. The benefits of the immunity that vaccines provide outweigh the serious risks associated with getting infected naturally. Wearing masks and social distancing help reduce the chance of being exposed to the virus or spreading it to others, but these measures are not enough. The COVID-19 vaccine is designed to work with immune systems so it will be ready to fight the virus if a person is exposed.
If a large portion of a community becomes immune to COVID-19 through vaccination, it can reduce the spread of the disease to others.
Will the COVID-19 vaccines be safe?
The new COVID-19 vaccines have been evaluated in tens of thousands of individuals who volunteered to be vaccinated and to participate in clinical trials using the same methods for many other U.S. Food and Drug Administration (FDA) approved vaccines currently in widespread use. The information from these clinical trials allowed the FDA to determine the safety and effectiveness of the vaccines. These clinical trials were conducted according to rigorous FDA standards. The FDA has determined that the newly authorized COVID-19 vaccines meet its safety and effectiveness standards; therefore, the FDA has made these vaccines available for use in the United States under what is known as an Emergency Use Authorization (EUA). The Centers for Disease Control and Prevention (CDC) and the FDA will continue monitoring the vaccines for safety issues after they are authorized and in use.
How effective will the vaccines be for disease prevention?
In Phase 3 trials, the Pfizer vaccine showed a 95% efficacy rate 7 days after the second dose. The vaccine was 94% effective in adults >65 years old.
The Moderna vaccine showed a 94% efficacy rate 14 days after the second dose. These results were consistent across gender, age, race, and ethnicity.
How do the Pfizer and Moderna mRNA vaccines work?
The vaccines contain synthetic mRNA, which is genetic information used to make the SARS-CoV-2 spike protein. The spike protein is the part of the virus that attaches to human cells. The spike protein alone cannot cause COVID-19.
Once the spike protein is created, it causes the immune system to make antibodies against the virus. These antibodies can then provide protection if a person comes into contact with the virus.
The mRNA vaccines are non-infectious and do not enter the human cell nucleus, so they cannot be inserted into human DNA. Additionally, mRNA is rapidly broken down, and this theoretically reduces chances for long-term side effects. The mRNA vaccines do not have the ability to cause cancer.
Learn more here: https://www.cdc.gov/vaccines/covid-19/downloads/healthcare-professionals-mRNA.pdf
Can I get COVID-19 from a vaccine?
No. None of the COVID-19 vaccines currently authorized for use or in development in the United States use the live virus that causes COVID-19.
Although some of the short-lived mild side effects such as fever, body aches, headaches from a COVID-19 vaccine are the same as COVID-19 infection, this does not mean that the vaccine causes infection. This means your immune system is working.
However, it should be noted that it typically takes a few weeks for the body to build immunity after vaccination. That means it’s possible for an individual to be infected with the virus that causes COVID-19 if exposed just before or just after vaccination. The vaccine itself, however, does not cause infection.
What is an Emergency Use Authorization (EUA)?
During a public health emergency, the FDA can use a process called “Emergency Use Authorization” (EUA) to allow the use of medical products that are not yet approved to diagnose, treat, or prevent serious or life-threatening diseases when certain criteria are met.
COVID-19 vaccines are currently being developed and tested for their safety and effectiveness in clinical trials (efficacy). Once vaccine manufacturers submit for authorization, the FDA evaluates the EUA request and determines whether they are safe and effective, taking into account the scientific evidence. For a vaccine to receive an EUA, the FDA must determine if the vaccine’s benefits outweigh its risks based on data from rigorous clinical trial(s).
Additional information on EUAs: https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
How does the FDA assess safety and effectiveness of a COVID-19 vaccine submitted for EUA?
COVID-19 vaccines are undergoing a rigorous development process that includes tens of thousands of study participants to gather required safety and efficacy data. FDA evaluates the information submitted by a vaccine manufacturer and uses all available tools and information, including records reviews, site visits, and previous manufacturing compliance history.
For an EUA to be issued, FDA must determine that the known and potential benefits outweigh the known and potential risks of the vaccine.
Link: https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
Which COVID-19 vaccines have been granted EUA?
The Pfizer COVID-19 vaccine received EUA on December 11, 2020 for individuals ages 16 and older. It is a 2-dose vaccination series, given intramuscularly, 21 days apart.
Why is vaccine development happening so quickly?
The vaccine process is happening faster because vaccine research and development, clinical trials, manufacturing, and plans for distribution are occurring at the same time. This method removes delays that occur when these processes are carried out one after the other. Steps to ensure safety are NOT being eliminated.
Why do we need a vaccine if we can take other COVID-19 precautions, like masking and physical distancing, to slow or prevent the spread?
It is vital that each person uses all tools available to stop the pandemic. Vaccines work with the immune system and allow a strengthened response to the virus if exposure occurs.
Other steps, like covering mouth and nose with a mask, washing hands, and staying at least 6 feet away from others, help reduce chances of exposure to the virus or spreading it to others.
Together, COVID-19 vaccination and following recommendations for self-protection and to protect others will offer the best prevention for further spread of COVID-19.
BEFORE GETTING VACCINATED
Are there any contraindications (conditions or factors that would be a reason to not get vaccination due to harm) to receiving the vaccine?
The only current contraindication to receiving the COVID-19 vaccine is anaphylaxis to any components of the COVID-19 vaccines. The vaccines are still being studied in pediatric populations and those under 16 years of age are not currently eligible for vaccination.
Should I take the COVID vaccine if I have a significant history of allergic reactions?
The CDC states severe allergic reaction to any vaccine or injectable therapy (intramuscular, intravenous, or subcutaneous) is a precaution to receiving vaccination. Vaccine providers should observe these patients for 30 minutes after vaccination to monitor for the development of immediate adverse reactions. This recommendation is due to two healthcare workers in the United Kingdom developing severe allergic reactions after receiving the vaccine. They both had a history of severe allergic reactions, carried epinephrine auto injectors, and fully recovered. Those with allergies to food, pets, insects, latex, or oral medications do not fall under this precaution and only have to be monitored for 15 minutes. Those with a history of severe allergic reactions should discuss this with their healthcare provider.
Should I take the vaccine if I am immunocompromised?
Currently, there is no data on the safety and efficacy of COVID-19 vaccines in immunocompromised people as they were excluded from clinical trials. However, people with immunocompromised conditions, or those on immunosuppressant medications, might be at increased risk for severe disease if they get COVID-19. Therefore, the CDC recommends these individuals receive the COVID-19 vaccine. Immunocompromised individuals should discuss this with their healthcare provider. It is important to note that the mRNA vaccines do not contain live virus, so it is not possible to develop COVID-19 from vaccination.
Should I take the vaccine if I am pregnant?
Currently, there is no data on the safety and efficacy of COVID-19 vaccines in pregnant women as they were excluded from clinical trials. However, it is known that pregnant women can have an increased risk of severe illness or negative pregnancy outcomes, such as preterm birth, if they become infected with COVID-19. Various experts during CDC and Advisory Committee on Immunization Practices meetings have commented that they believe the vaccine is unlikely to cause placental and fetal exposure and that there is a minimal safety risk as the mRNA vaccine is not a live vaccine. Reputable sources, such as the American College of Obstetricians and Gynecologists, have advised that the benefit of vaccination may outweigh the risk of severe COVID-19 disease. For this reason, if a pregnant woman is part of a group who is recommended to receive a COVID-19 vaccine, she may choose to be vaccinated. A discussion with her health care provider can help her make an informed decision.
Should I take the vaccine if I am breastfeeding?
Currently, there is no data on the safety and efficacy of COVID-19 vaccines in breastfeeding women as they were excluded from clinical trials. However, the CDC has stated that since the mRNA vaccine does not contain live virus, it is not thought to be a risk to breastfeeding infants. For this reason, if a breastfeeding woman is part of a group who is recommended to receive a COVID-19 vaccine, she may choose to be vaccinated. A discussion with her health care provider can help her make an informed decision.
Are the mRNA vaccines safe for a woman who wants to become pregnant?
There is no evidence the COVID-19 vaccine affects fertility. Women who are trying to become pregnant or who are pregnant and for whom the vaccine is recommended may choose to be vaccinated. A discussion with her health care provider can help to make an informed decision.
Should I take the vaccine if I have had convalescent plasma or monoclonal antibody?
Currently, there is no data on the safety and efficacy of COVID-19 vaccines in people who received convalescent plasma or monoclonal antibody therapy. Vaccination should be deferred until 90 days after receiving convalescent plasma or monoclonal antibodies. This is to avoid interference of these treatments with vaccine-induced immune responses.
Should I take the vaccine if I already had COVID-19 and recovered? How long after should I take it?
Those who have had COVID-19 and recovered should still receive the vaccine. The length of immunity after recovering from COVID-19 is unknown; early studies show that it is not long lasting and rare cases of reinfection have been reported. The Pfizer trial did include a small percentage of individuals who previously had COVID-19 and recovered. The CDC states current evidence suggests reinfection is uncommon within 90 days after initial infection, so vaccination can be deferred until the end of this period; however, it is not known when another vaccination will be available to you. For this reason it is recommend to taking the vaccine once in the COVID-19 isolation period ends and it is available.
Should I take the vaccine if I currently am infected with COVID-19?
No. Those infected should wait until they are no longer in isolation.
Should I get the vaccine if I am in quarantine?
Community or outpatient setting: Defer vaccination until quarantine period has ended to avoid exposing healthcare personnel (HCP) or other persons during vaccination visit.
Residents of congregate healthcare settings (e.g., long-term care facilities) may be vaccinated, as this likely would not result in additional exposures. HCP are already in close contact with residents and should employ appropriate infection prevention and control procedures.
Residents of other congregate settings (e.g., correctional facilities, homeless shelters, residential settings) may be vaccinated in order to avoid delays and missed opportunities for vaccination. Where possible, precautions should be taken to limit mixing of these individuals with other residents or non-essential staff.
How long after the flu shot do I have to wait to take the COVID-19 vaccine?
Wait a minimum of 14 days after receiving the flu shot or any other vaccine to receive a COVID-19 vaccine. The safety or efficacy of taking the COVID-19 vaccine at the same time as other vaccines is currently unknown.
Should premedication be given prior to vaccination?
Taking medications such as acetaminophen or ibuprofen before receiving the vaccine to try to prevent side effects like fever or pain is not recommended at this time. This is because there is not enough information on how pain-relieving medications will impact antibody responses. These medications can be taken after receiving the vaccine if side effects present.
GETTING VACCINATED
What is the Vaccine Administration Management System (VAMS)?
The Vaccine Administration Management System (VAMS) is an easy-to-use, secure, online tool to manage vaccine administration from the time the vaccine arrives at a clinic to when it is administered to a person. VAMS is free for public health-approved clinics and can be used on computers, tablets, and other mobile devices. It is not a smartphone app; no installation or download is required for this web-based platform.
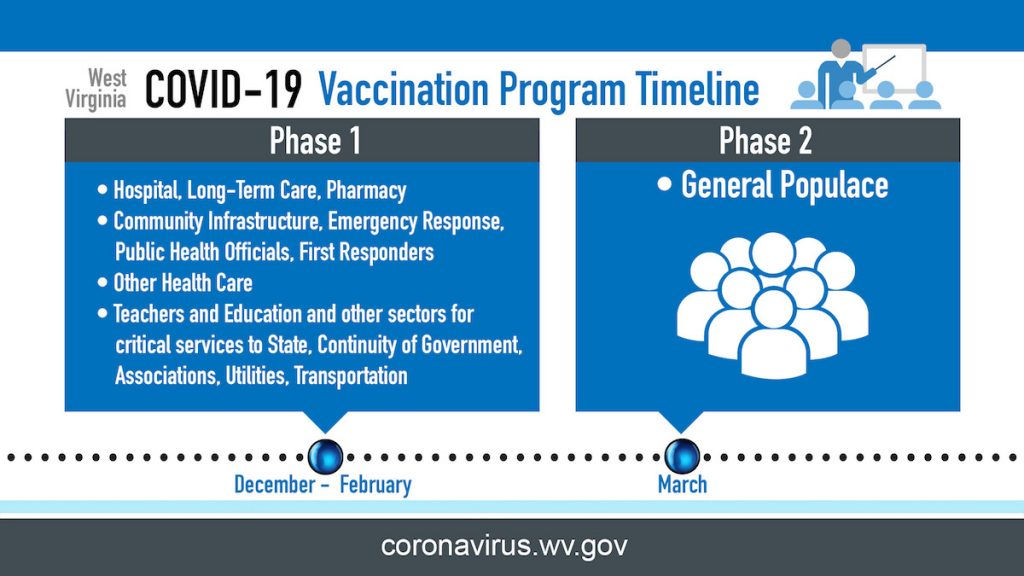
West Virginians in Phase 1 will use VAMS to schedule their vaccination appointment. Phase 1 participants may receive an email from [email protected] to schedule the appointment. Appointments may also be scheduled by an employer or long-term care facility.
For more information on VAMS: https://www.cdc.gov/vaccines/covid-19/reporting/vams/faqs.html
When will I get the vaccine?
Pfizer vaccine was granted EUA on December 11, 2020 and an independent advisory committee to the CDC recommended its use. A limited supply of Pfizer vaccine will arrive in West Virginia the week of December 14, 2020.
Moderna vaccine is under review by the FDA for EUA.
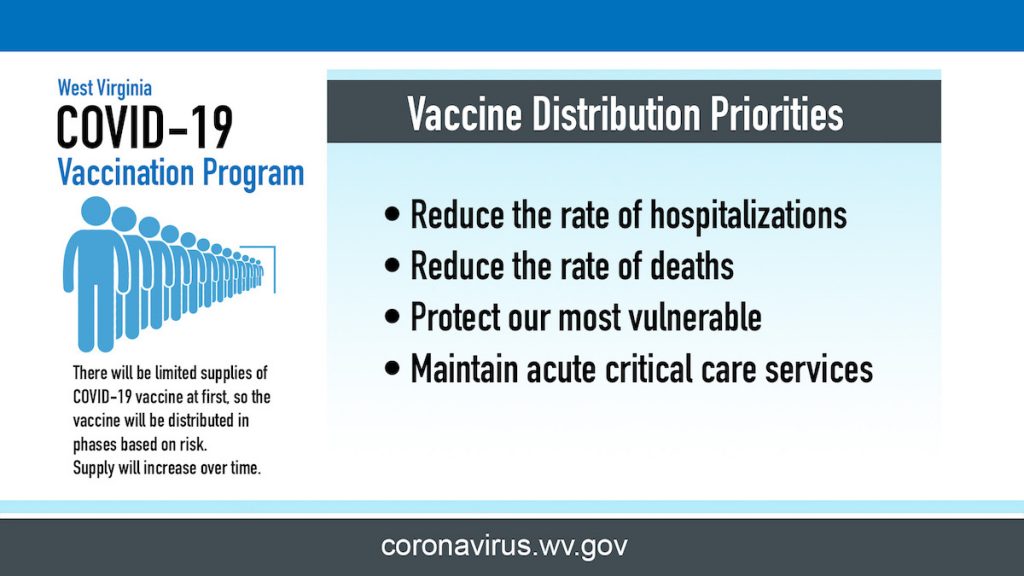
West Virginia is dedicated to ensuring that all West Virginians have access to a COVID-19 vaccination as soon as possible. The initial focus in vaccine distribution is to take care of the most vulnerable residents. Vaccines will be in limited supply at the beginning, so the first phase will be distributed to individuals in high-risk settings such as healthcare, emergency response, long-term care facilities, and community infrastructure. This approach is imperative to preserve critical infrastructure, such as making sure the West Virginia’s healthcare system can meet the state’s needs.
As West Virginia receives more vaccine supply and has vaccinated those identified for Phase 1, vaccinations will be made available for the general public.
How is the COVID-19 vaccine administered?
COVID-19 vaccines are given through intramuscular (IM) injections.
Who will administer the COVID-19 vaccine?
The vaccine will be administered by a health care professional trained in giving an injection into the muscle.
How long between Pfizer vaccine doses? What happens if I’m late for the second dose?
The Pfizer product requires a two-dose vaccination series administered 21 days apart. Administration of 2nd dose is allowed within a 4-day grace period (meaning days 17-21). If more than 21 days have passed since the 1st dose, the 2nd dose should be administered at the earliest opportunity to avoid repeating doses.
For 2-dose vaccines, what happens if I only receive one dose of the vaccine and not both?
It is recommended to receive both doses of the vaccine for maximum protection.
How will the second dose of the vaccine be ensured?
The Vaccine Administration Management System (VAMS) will allow clinicians to set up customized vaccine schedules and allow recipients to make vaccination appointment, in addition to sending a reminder about returning for a second dose if required.
Is taking the COVID-19 vaccine mandatory?
The vaccine is not mandatory; however, it is recommended in order to help prevent disease spread and reduce severity. Getting vaccinated will improve the health and well-being of West Virginia communities and help the economy.
AFTER GETTING VACCINATED
What are the Pfizer COVID-19 vaccine side effects?
Short-Term: In the Pfizer vaccine clinical trial, the majority of short-term side effects reported were mild to moderate, short-lived, and occurred within the first few days of receiving the vaccine. Examples of common mild to moderate side effects include pain at the injection site, headache, fatigue, fever, or chills. Side effect occurrence is typically higher after the second dose of vaccine.
Long-Term: Historically, long-term side effects from vaccines have been rare and most side effects have been seen within the first 60 days of receiving vaccines.
How will side effects from the vaccines be treated?
Side effects from vaccines are typically short-lived. If you are concerned about your health after getting vaccinated, talk with your health care provider. They will determine the appropriate treatment. You or your doctor can choose to report the side effect to the Vaccine Adverse Event Reporting System (VAERS): https://vaers.hhs.gov/.
If you develop COVID-19 symptoms after getting the vaccine, should you quarantine?
Yes. It typically takes a few weeks for the body to build immunity after vaccination. That means it is possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and get sick. This is because the vaccine has not had enough time to provide protection for disease.
If you have COVID-19 virus symptoms after getting the vaccine or at any time, contact your health care provider and consider getting tested for COVID-19.
Do you need to quarantine if you are exposed between vaccine doses?
If exposure occurs between doses, follow quarantine guidance as advised by the local health department.
Quarantine is used to keep someone who might have been exposed to COVID-19 away from others. Quarantine helps prevent spread of disease that can occur before a person knows they are sick or if they are infected with the virus without feeling symptoms.
What is the V-safe After Vaccination Health Checker?
V-safe is a smartphone-based tool that uses text messaging and web surveys to provide personalized health check-ins after a person receives a COVID-19 vaccination. Through V-safe, a person can quickly tell the CDC if they experience side effects after getting the COVID-19 vaccine. Depending on the person’s responses, a CDC staff member may call for additional information. V-safe also sends reminders to get the second COVID-19 vaccine dose. Participation in the CDC’s V-safe initiative makes a difference — it helps keep COVID-19 vaccines safe.
For more information on V-safe: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html
How long will immunity last after I get vaccinated? Will I need to be vaccinated every year?
The length of immunity following vaccination is not yet known for COVID-19.
Do I still need to wear a mask and take other COVID-19 precautions after I get the vaccine?
Yes. Wearing a mask, washing hands, and staying at least 6 feet away from others will remain important after receiving the vaccine. Because there will be limited doses available initially, and people will be vaccinated in waves, it will take time to vaccinate enough of the population to stop the spread of COVID-19.
Additionally, as the length of immunity is unknown, infection after a receiving a vaccine may still be possible. It is likely that infection after receiving the COVID-19 vaccine would be less severe, with mild or asymptomatic conditions.
Other factors for continuing precautions include how many people get vaccinated and how the virus is spreading in West Virginia communities.
How many people need to get the vaccine for community immunity (herd immunity)?
Vaccination is the safest path to community or “herd” immunity. These terms describe when enough people have protection, either from previous infection or vaccination, making it unlikely a virus or bacteria can spread and cause disease. As a result, everyone within the community is protected even if some people haven’t received the vaccination. The percentage of people who need to have protection in order to achieve community immunity varies by disease. The number or percentage of population that need to be vaccinated in order to reach community immunity for COVID-19 is not yet known.





